Patients
LIVERATION’s aim is to lower the chances of liver cancer coming back after surgery in the surgical bed, improve survival rates, and enhance quality of life for people with colorectal cancer with liver metastasis or hepatocarcinoma. We also want to learn more about how patients feel, and what they want and need while going through the surgery and recovery process.
If you are interested in participating in the clinical trial, please contact a participating hospital in your country for more information here.
For patients who are already enrolled in the clinical trial, below we provide information on how to prepare for surgery, what to expect on the day of the surgery and during the hospital stay, and what to expect after the surgery.
Patients and other stakeholders’ involvement in LIVERATION
If there is other information you need or resources you would like us to share, please feel free to contact us.
Patients and other relevant stakeholders can also get involved in the project by participating in the European-level Multistakeholder Group or the Living Labs.
Ultimately, LIVERATION findings will inform the development of an Interactive Decision Tool to be used by patients and professionals that aims to help guide the decision-making process.
This page will be continuously updated based on stakeholders’ feedback.
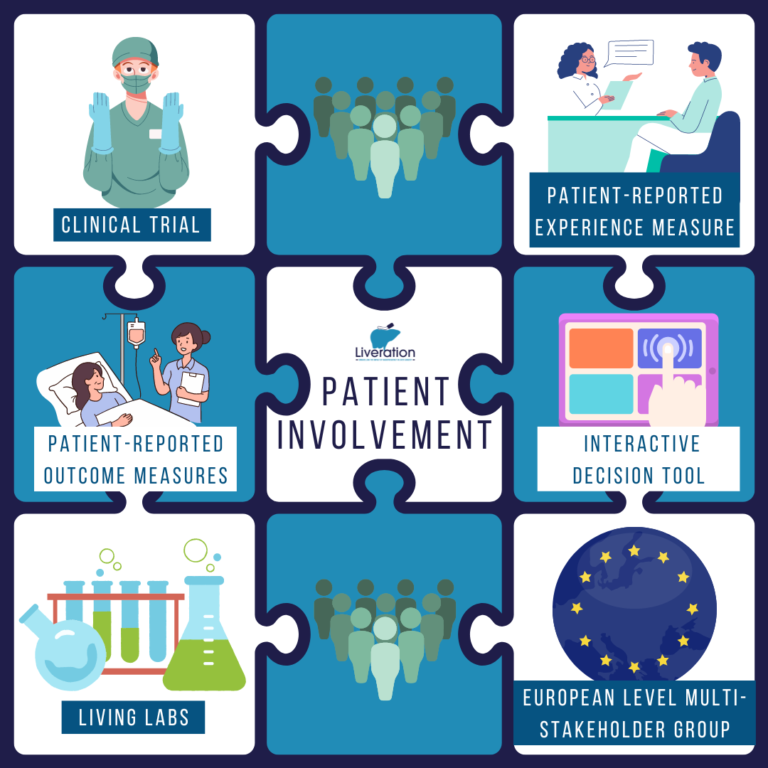
European-level Multistakeholder Group
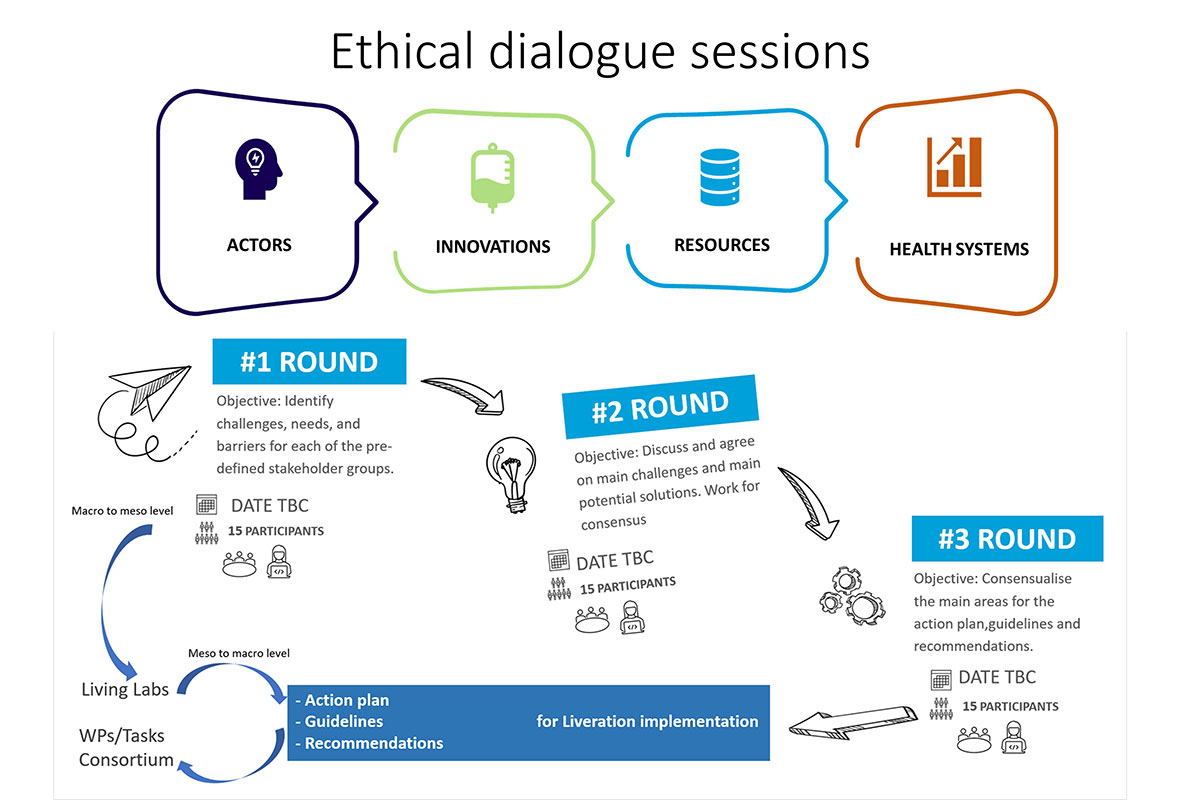
We developed a detailed stakeholder analysis to define and establish LIVERATION’s European multi-stakeholder group.
It will involve citizens and patients, family and caregivers, health and care professionals, industry, hospital managers, ethicists, public authorities and regulators in three online iterative discussions and co-creation activities.
More information here
- Round 1: We will begin by understanding the challenges, needs, and obstacles faced by each of the stakeholder groups we’ve identified.
- Round 2: Next, we will collectively discuss and agree upon the main challenges and potential solutions.
- Round 3: Finally, we will work together to reach a consensus on the primary areas that will be addressed through our action plan, guidelines, and recommendations.
Are you a potentially relevant stakeholder for our group and want to join us?
Please contact us here and we will further share with you how to apply!
Living labs
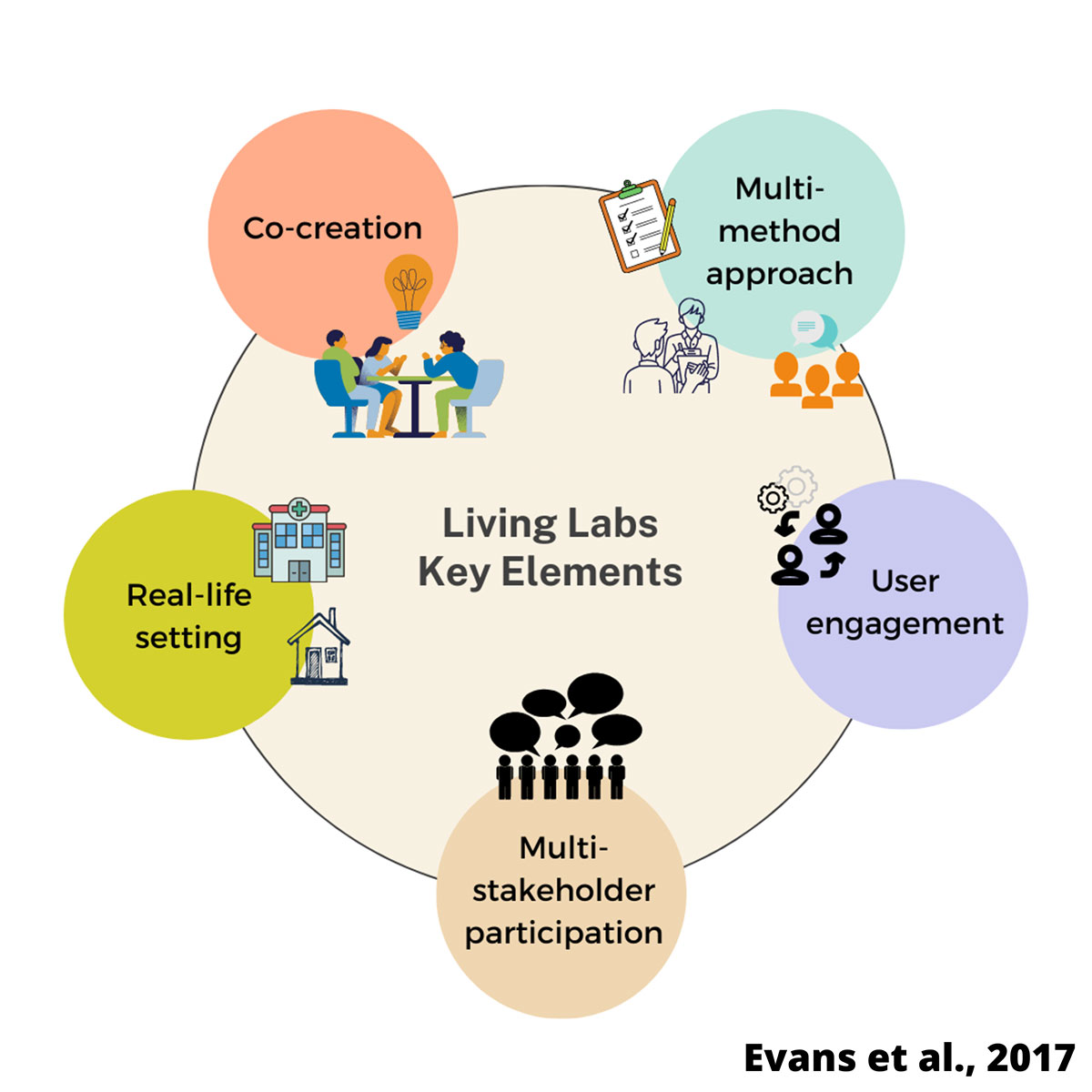
Living labs are creative spaces where different groups of stakeholders come together to co-create innovations that will address current needs in real-world contexts (U4IoT Consortium, 2017).
More information here
During LIVERATION, we aim to co-create clinical living labs in one hospital per participating country to identify and prioritize end-user needs and preferences related to quality of life, patient experience, and stakeholder engagement. We will integrate findings from.
- The patient-reported health-related quality of life and experience questionnaires.
- Interviews with patients, patient representatives, caregivers, professionals and other relevant stakeholders, and co-design groups.
- Findings from the living lab design process will be shared with the European-level multistakeholder group to attain feedback and vice versa.
Interactive Decision Tool
Timeline: 2026 – 2028
Healthcare decision aids help inform patients and professionals about different evidence-based healthcare options to tailor clinical decisions to individual patient’s needs and preferences. They support evidence-based decision making and have been shown to increase patient knowledge.
More information here
Download our Patients leaflet
The interactive decision tool will be developed based on previous LIVERATION findings including the results from a systematic review of the literature, the clinical trial, patient-reported health-related quality of life and patient experience, the European-level multistakeholder group and the living labs.

