The LIVERATION Project
Colorectal cancer is the third most common tumour in men and the second in women, with an forecast of 1.9 million new cases worldwide (450K in Europe) and approximately 900,000 deaths in 2020. Hepatic metastases develop in around 50% of colorectal cancer cases.
Furthermore, the liver is the first and only area of spread in 30–40% of patients. Surgery is considered the gold standard treatment and the only potentially curative option for colorectal liver metastases.
LIVERATION Videopills
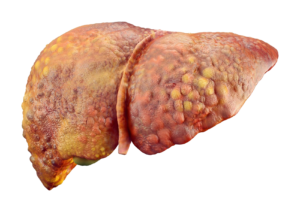
Patients who are diagnosed at an early stage without metastasis of the disease are candidates for treatments including surgical resection, ablation, trans-arterial chemoembolization and liver transplantation, and have a 5-year overall survival between 50-70%.
Furthermore, even after radical resections, hepatocellular carcinoma still shows a high recurrence rate (five-year recurrence rates ~70% postresection, with 2/3 of recurrences occurring within 2 years related to intrahepatic spread and a rate of local recurrence about 20%).
On the other hand, the major proportion of patients who present with hepatocellular carcinoma are initially not appropriate candidates for surgical resection (is recommended only for patients with a single lesion without clinically significant portal hypertension and with a future liver remnant greater than 40% to maintain adequate liver function).


LIVERATION leverages a prospective pragmatic clinical trial coordinated by expert clinicians and surgeons on liver cancer that had achieved promising preliminary results: a reduction of cancer local recurrence by adding an additional coagulation of the margin (ACM) with radiofrequency (RF) ablation devices compared to conventional hemostatic techniques in a retrospective clinical trial.
LIVERATION OBJECTIVE
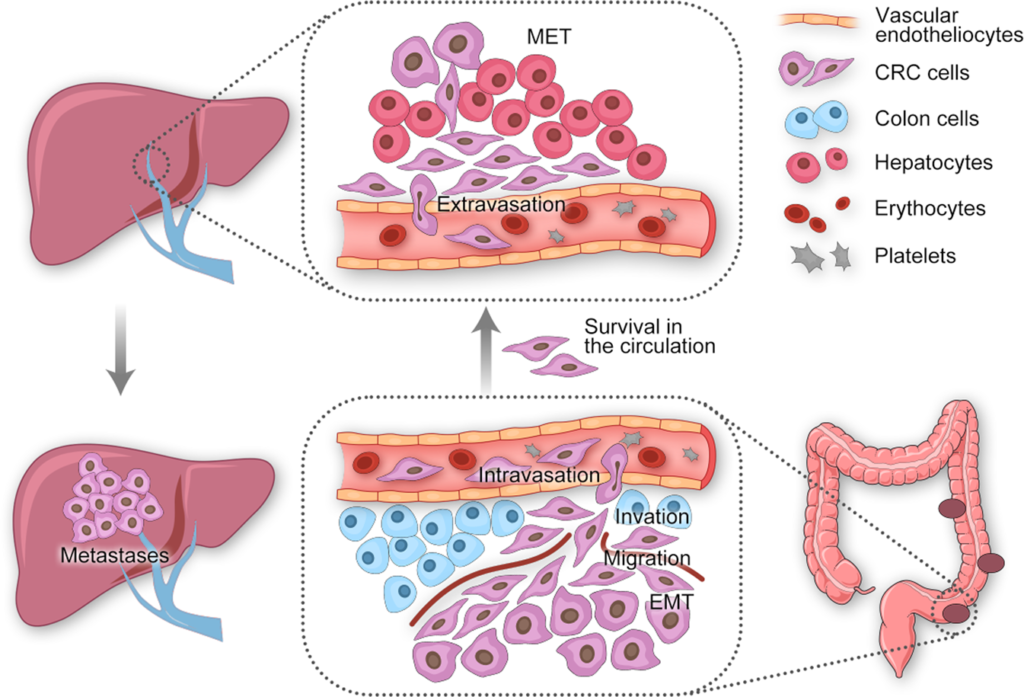
1) the portal vein system directly connects the colorectal region and liver, which is associated with abundant blood supply.
2) location and histological type of primary tumour.
LIVERATION Methodology
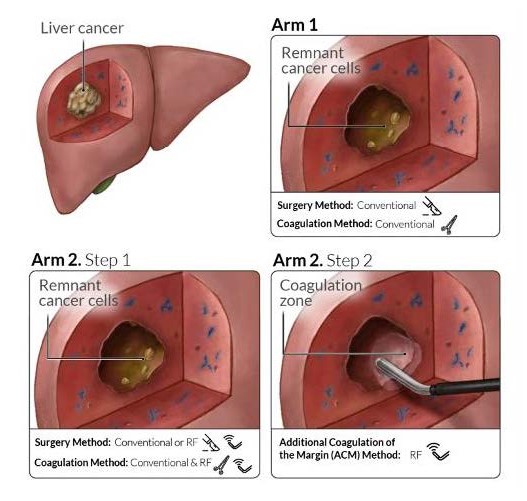
In the arm 1 of the clinical study, conventional (no ablative) methods of haemostatic resection (such as Conventional crush-clamp or finger fracture technique, Ultrasonic dissector (e.g CUSA) or Ultrasonic mediated devices, Water jet dissectors, Argon beam coagulator and Bipolar forceps) will be used. These technologies aimed for arm 1, might be used by the chosen centres at their preference after accurate randomization of the patients between arm 1 and arm 2 methodology will be used causing a specific additional coagulation of the tumour margins with a radiofrequency-assisted device (Coolingbis or Aquamantys) in the liver after resection and transection of the tumour.
LIVERATION WORKING PACKAGES (WPs)
WP1: Management and coordination.
1) To establish and guarantee full synergy, motivation, and effective interactions among LIVERATION participants, coordinate their action towards full successful execution of the Project tasks in a timely fashion and within the budget and quality limits as defined by the Grant Agreement signed with the EC and the Consortium Agreement signed among the beneficiaries and those participating as sites.
2) Ensure efficient operational management including that of administrative, financial and legal issues. The Project coordinator is Patricia Sánchez Velázquez and the Hospital del Mar/IMIM institute.
